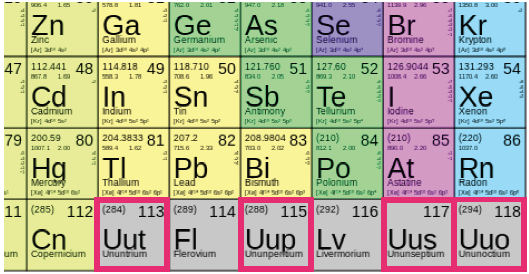
At the end of last month, the International Union of Pure and Applied Chemistry announced that four new elements would officially be added to the periodic table. This means that the seventh period, or row, of the table is finally complete.
Element 113 was created by the RIKEN research institution in Japan, while the other three elements were created by collaborative efforts between Russian and American scientists.
All four of the new elements are synthetic, meaning that they were created in labs and do not exist naturally.
However, according to Richard Longland, an assistant professor in the NC State Department of Physics, “It’s likely that merging neutron stars produced similar high-mass elements in our own solar system’s ancestral past.”
In describing the significance of the discoveries, Longland said, “These groups [of scientists] are at the forefront of discovery. Their findings affect our fundamental understanding of how nature’s building blocks, protons and neutrons, were arranged to produce the solar system as we know it today.”
The newly recognized elements, with atomic numbers 113, 115, 117 and 118, are still yet to be named. According to Chemical and Engineering News Magazine, the IUPAC hopes to have the names finalized within the next five to six months.
“Elements are typically named after who discovered them or synthesized them, something about the place of their discovery, or something about the element,” said Alton Banks, a chemistry professor at NC State.
The right to name the elements is left to the discoverers, or in this case, the creators. The task of creating elements is a daunting one and requires smashing atoms together to create larger atoms.
“[The new elements] were discovered by smashing two lower mass atoms together,” Longland said. “The probability of them fusing is tiny; the probability of them fusing without boiling off stray protons and neutrons is tinier still.”
Banks explained the process using baseball as an example.
“It’s just a group of people tossing high energy particles at much larger atoms,” Banks said. “It’s very much like trying to pitch a baseball at a catcher’s mitt so fast that the baseball and the catcher’s mitt merge. And when they do, they form a new element.”
Although the elements are being recognized now, it is likely that their significance will not be felt until much later. This is because the elements are difficult to produce, and many of their properties are not yet known.
“The researchers only make one or two atoms at a time, and the atoms only live a second or less before decaying,” Christopher Gould, a professor in the physics department at NC State, said. “It will be a long time before anyone does any chemistry with them.”
CEN Magazine quoted Dawn Shaughnessy, the principal investigator for the Heavy Element Group at Lawrence Livermore National Laboratory, as saying, “The periodic table says that [element] 118 is a noble gas. How could we determine that based on a single atom?”
Despite the elements’ lack of current uses, chemists and physicists may find use for them in the future. After all, Americium, an element that was discovered in 1945, is today used in smoke detectors to detect fires.
“For me to sit here and say that the four new elements aren’t going to be of any use is ignoring history, and I try not to do that,” Gould said. “I think that the discovery of these four new elements points to the fact that there is a need for continuing research.”